A plastic printer can be converted into a bioprinter
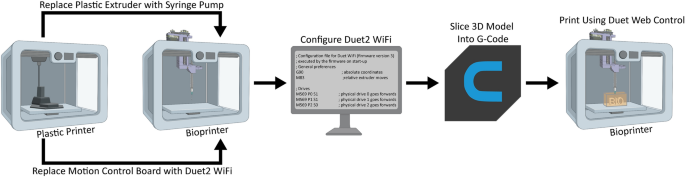
[****]There are several steps involved in converting a 3D printer made of plastic into a bioprinter. These steps generally follow the same sequence (Fig. 1). The electronics and control system for the plastic printer must be modified to allow bioprinting. The FlashForge Motion Control Circuit Board (Fig. 2A, the green rectangle) has been replaced by the open-source Duet 2 WIFI motion control circuit board (Fig. 2B, blue rectangle). This is done to increase the motion control performance, enable WiFi access, and facilitate quick firmware customization via the Duet web-based interface. These instructions are for FlashForge Finder. They can also be adapted to most desktop 3D printers (details in Supplementary Figs). S1–S15, and provided Duet2 WiFi configuration files). The thermoplastic extruder included with the printer will be replaced by the Replistruder 4 which is an open-source, high performance syringe pump extruder.14. The majority of the parts of Replistruder 4 can be 3D printed from plastic and assembled with commonly available hardware (Fig. 2C). A carriage platform was designed to fit onto the existing linear motion components and provide a mounting point. This carriage for the X axis has pockets to mount the bearings on the X axis linear rails. It also includes channels for routing and maintaining the X axis belt. To allow the Replistruder 4 attachment to the Xaxis carriage, four mounting points have been provided with recessed M3 hexagon nuts (Fig. 2D). The thermoplastic printhead that comes pre-installed on the Finder is replaced with the X-axis carriage/Replistruder 4 assembly (Fig. 2E,F, more details in Supplementary Figs. S16–S23). The Replistruder 4 is ready for use after these steps are completed (Fig. 2G, blue Arrow) is mounted in the X axis carriage of the printer (Fig. 2G, yellow arrow) is mounted on the X-axis of the printer in the X-axis carriage (Fig. 2H, green arrow). These modifications transform the FlashForge Finder into an open-source bioprinter that features a high-performance extruder with motion control and a high-performance extruder.
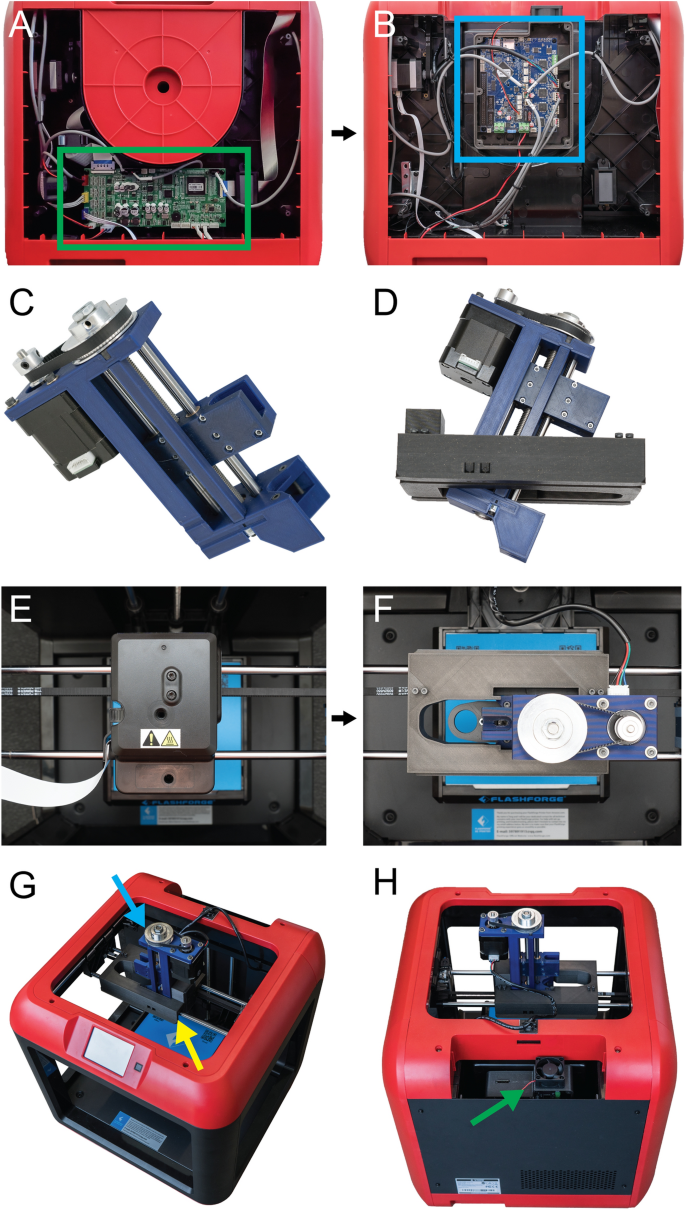
[****]Duet 2 WiFi has several key advantages over the FlashForge Finder stock motion control boards and other low-cost desktop 3-D printers. First, the Duet’s WiFi based web interface allows for easy, in-browser access to printer movement, file storage and transfer, configuration settings, and firmware updates. This is unlike most 3D printers which require you to flash the firmware of the motion control boards using third-party software. For inexperienced users, this can be difficult and intimidating and could lead to firmware corruption or accidental changes. Second, the Duet 2 adds many advanced motion control improvements including (i) a 32-bit electronic controller, (ii) high performance Trinamic TMC2260 stepper controllers, (iii) improved motion control with up to 256 × microstepping for 5 axes, (iv) high motor current output of 2.8 A to generate higher power, (v) an onboard microSD card reader for firmware storage and file transfer, and (vi) expansion boards adding compatibility for 5 additional axes, servo controllers, extruder heaters, up to 16 extra I/O connections, and support for a Raspberry Pi single board computer. You can also access the Duet 2 WiFi setup online. The documentation is updated regularly and is supported by an active user community. The Duet2 WiFi setup guide and the hardware details are provided. However, the official Duet3D documentation can be accessed for those who wish to modify the Duet2 WiFi. This combination provides high precision motion control and expandability. It also includes an easy-to use web interface that allows rapid customization and improves performance over standard desktop plastic printers.
Mechanical performance of a 3D bioprinter
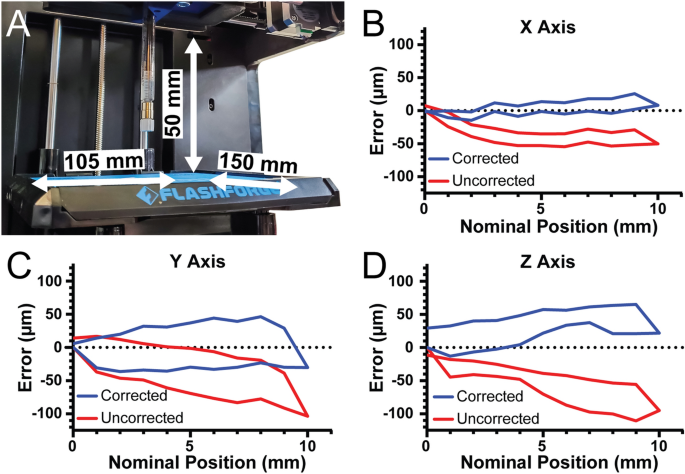
[****]To determine the 3D bioprinter’s build volume, the X-, Y-, and Z axis travel limits were determined after conversion. For the X-axis travel is 105 mm, for the Y-axis travel is 150 mm, and for the Z-axis travel is 50 mm, resulting in an overall build volume of 787.5 cm3 (Fig. 3A). The most critical control parameter for a stepper motor-driven motion system such as this 3D bioprinter or most commercial 3D printers is the steps per millimeter calibration for each of three axes. This number tells the stepper motors how many pulses or steps must be sent each axis in order to move them exactly one millimeter. The formula for the X- and Y-axes, which have belt drive, is (steps/mm=(steps/rotationtimes microsteps)/(belt pitchtimes pully teeth)). For the Finder these parameters are the nominal pitch of the driving belt (2 mm), the number of teeth in the motor’s pully (17 teeth), the number of steps in a full rotation for the motor (200 steps), and the number of microsteps that the Duet 2 WiFi interpolates between the full steps (set to 64 microsteps). The nominal steps/mm of the X- and Y-axes are 376.5. The formula for the Z-axis is (steps/mm=(steps/rotationtimes microsteps)/(screw pitchtimes screw starts)). The finder uses a 4 start, 2 mm pitch lead screw so the nominal steps/mm for 16 × microstepping is 400 steps/mm.
[****]The key specifications for 3D bioprinter performance are those that directly affect print quality. Many thermoplastic 3D printers include specifications for resolution. This refers to the smallest steps the printer can take in any given direction. Table 1 shows the numbers reported for the FlashForge Finder, as well as several other popular bioprinters. Additional details regarding the motion system include positional error (the absolute deviation of the current location for the FlashForge Finder from the intended location) and repeatability (the maximum absolute deviation in measured positions from the average measured location when trying to reach the position multiple times). These metrics are not commonly used in bioprinting and may vary depending on the mechanical components and assembly quality. The resolutions provided by bioprinter specifications are based on the nominal dimensions and the screws used to assemble the system. None of these manufacturers offer measurements of actual resolution. This is either error over the entire distance traveled or repeatability. These measurements are often provided by ultra-high-end motion platforms like those offered by Aerotech and Physik Instrumente.28,29. Measure the travel distance with an external tool to optimize these low-cost 3D printers.
[****]To verify that the nominal steps/mm values were correct, we quantified positional error of our system along each axis near the center of travel with 2 µm precision. The nominal steps/mm values showed a systematic undertravel for the X-axis (Fig. 3B, red curve. Using the maximum error at 10 mm of travel we determined the number of missed steps per mm and corrected the value, and with this corrected steps/mm the average travel error over the 10 mm window was 7.9 µm (Fig. 3B, blue line. The Y-axis was subject to systematic under-travel according to the nominal steps/mm (Fig. 3C, red curve) and after correction this was reduced to 29.1 µm (Fig. 3C, blue contour. Finaly, the Z-axis was subject to systematic under-travel according to the nominal steps/mm (Fig. 3D, red curve) and after correction was reduced to 32.3 µm (Fig. 3D, blue curvature. The values also enable calculation of the unidirectional repeatability, which is the accuracy of returning to a specific position from only one side of the axis (e.g., from 0 to 5 mm) and the bidirectional repeatability, which is the accuracy of returning to a specific position from both sides of the axis (e.g., from 0 to 5 mm and from 10 to 5 mm). For the X-axis, the unidirectional repeatability was 3.9 µm, and the bidirectional repeatability was 16.4 µm. For the Y-axis, the unidirectional repeatability was 11.5 µm, and the bidirectional repeatability was 63.9 µm. For the Z-axis, the unidirectional repeatability was 8.7 µm, and the bidirectional repeatability was 38.7 µm. Together these measurements demonstrate that with calibration the travel of our converted bioprinter had a maximal error of 35 µm and repeatability in worst case situations of 65 µm. Whereas before calibration there was a linearly increasing error in position, after calibration this error is significantly decreased. It would be difficult to tell if defects in printed constructions are due to flaws in the printer or other factors that impact print quality without this calibration or at least measuring the errors.
Bioprinter printing fidelity:
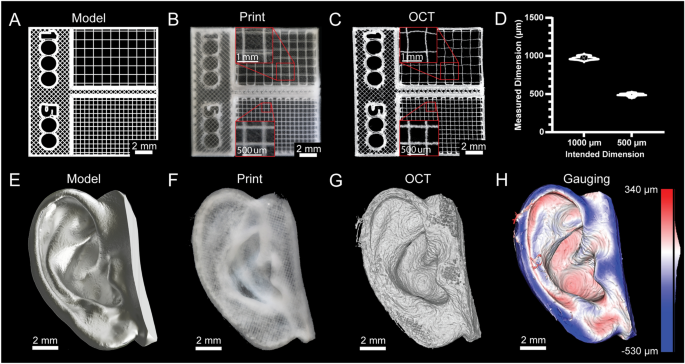
[****]Fidelity and resolution of printed constructs are typically not quantified for 3D bioprinters because they cannot print bioinks in a manner approaching the mechanical limitations of the systems. However, advances in embedded bioprinting techniques like FRESH have allowed for some remarkable improvements.13, it is now possible to perform extrusion bioprinting with resolutions approaching 20 µm. Thus, to demonstrate bioprinter printing performance we generated a square-lattice scaffold design consisting of 1000 and 500 µm filament spacing (Fig. 4A) To measure the accuracy when FRESH printing with a collagen type 1 bioink (Fig. 4B). 3D volumetric images were taken using optical coherence imaging (OCT) to measure grid spacing (Fig. 4C)30This revealed a good agreement between the measured dimensions and those as-designed (Fig. 4D). The next step was a more complicated design that was based on 3D scanning of the adult human ear (Fig. 4E). This model was printed with collagen (Fig. 4F). We used OCT to capture a 3D volumetric image (Fig. 4G, Supplementary Figure. S24)30. The 3D reconstruction demonstrates recapitulation of the features of the model and 3D gauging analysis revealed a deviation of − 29 ± 107 µm (mean ± STD) between the FRESH printed ear and the original 3D model (Fig. 4H)30. These two examples together show that the average error (and standard deviation) of the printed scaffolds were within the mechanical limits of the bioprinter we constructed, and comparable with commercial bioprinters.31.